Alkaline Ionized Water
What is your body's desired water, alkaline water?
SCROLL
80% of our bodies are water!
Then what kind of water
would you like to drink?
Then what kind of water
would you like to drink?

Alkaline Ionized Water absorbs quickly into the body
because the molecules are smaller than ordinary water.
It is effective in improving the four major gastrointestinal symptoms,
and the body's cells are weakly alkaline,
so it helps the growth, regeneration, and production of cells.
because the molecules are smaller than ordinary water.
It is effective in improving the four major gastrointestinal symptoms,
and the body's cells are weakly alkaline,
so it helps the growth, regeneration, and production of cells.
What is Healthy water, good water ?
| 1 | The water should taste good because it contains a balanced amount of minerals that are beneficial to the body. |
|---|---|
| 2 | There should be no chemicals or harmful substances such as chlorine, rust, bacteria, and E. coli. |
| 3 | Alkaline water, like the human body, keeps you healthy. |
| 4 | The ability to remove free oxygen must be high due to the abundance of free hydrogen. |
| 5 | Small, dense water must be used for quick absorption and good water tastes good. |
As the voltage between the
anodes increases, the positive
surface is electrolyzed to produce
alkaline and acidic water.
anodes increases, the positive
surface is electrolyzed to produce
alkaline and acidic water.

What is pH?
pH stands for "potential of hydrogen" and is a unit of measurement that
expresses the concentration of hydrogen ions in water.
The pH can represent acidic, neutral, and basic aqueous solutions in simple numbers. pH of the acidic solution is less than 7,
The pH of the neutral solution is 7, and the pH of the basic solution is greater than 7. Based on the pH of drinking water in Korea, it is 5.8 to 8.5 and
The pH of fresh water where fish live is 6.7-8.6 Acid rain is rainwater with a pH of 5.6 or less.
Rainwater contaminated by sulfur dioxide or nitrogen dioxide shows pH below 5.6 and is called acid rain.
The pH can represent acidic, neutral, and basic aqueous solutions in simple numbers. pH of the acidic solution is less than 7,
The pH of the neutral solution is 7, and the pH of the basic solution is greater than 7. Based on the pH of drinking water in Korea, it is 5.8 to 8.5 and
The pH of fresh water where fish live is 6.7-8.6 Acid rain is rainwater with a pH of 5.6 or less.
Rainwater contaminated by sulfur dioxide or nitrogen dioxide shows pH below 5.6 and is called acid rain.
pH values of solutions commonly
found in daily life.
found in daily life.
| TYPE | PH |
|---|---|
| The acid used in batteries | 0.1 ~ 0.3 |
| gastric juice | 1.0 ~ 3.0 |
| vinegar | 2.4 ~ 3.4 |
| Carbonated drinks | 2.5 ~ 3.5 |
| TYPE | PH |
|---|---|
| Drinking water | 6.3 ~ 6.6 |
| Pure water | 7.0 |
| seawater | 7.8 ~ 8.3 |
| Ionized water | 7.5 ~ 10.0 |
| Ammonia solution | 10.6 ~ 11.6 |

pH experiment
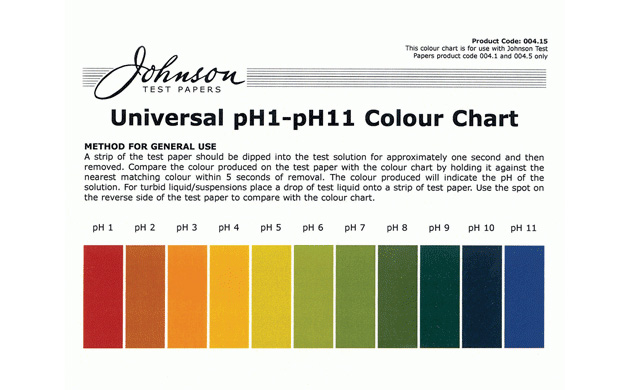
pH color chart
What is ORP?
When we look at the structure of an atom, there is a positively charged nucleus in the center, and negatively charged electrons orbiting around it. If the electrons are paired, the atom is stable. However, if there is an odd number of electrons, the atom tries to stabilize by taking or giving away electrons from other atoms, creating a cycle of oxidation and reduction reactions. The act of losing electrons is called oxidation, while the act of gaining electrons is called reduction.
egree of oxidation and reduction is measured by a value called the Oxidation-Reduction Potential (ORP). The higher the ORP value, the more oxidized the substance is, and the lower the reduction potential, the stronger the substance is in returning to its normal state from an oxidized state.
egree of oxidation and reduction is measured by a value called the Oxidation-Reduction Potential (ORP). The higher the ORP value, the more oxidized the substance is, and the lower the reduction potential, the stronger the substance is in returning to its normal state from an oxidized state.
Comparison chart of ORP values
| TYPE | PH |
|---|---|
| Ionized water | +50mV ~ -500mV |
| Newborn baby's body fluid | +100mV ~ -300mV |
| Healthy adult's body fluid | +100mV ~ -100mV |
| Spring water, deep sea water | +200mV ~ -100mV |
| TYPE | PH |
|---|---|
| Bottled water | +300mV ~ +200mV |
| Tap water | +700mV ~ +500mV |
| Regular air | +800mV ~ +700mV |
| Activated oxygen | +800mV or higher |
FEATURES
01
Maintains alkalinity
PH
The body fluid has a
slightly alkaline pH
of approximately 7.38 to 7.45.
slightly alkaline pH
of approximately 7.38 to 7.45.
Homeostasis
Body's processes to maintain
a slightly alkaline pH
in the body fluid.
a slightly alkaline pH
in the body fluid.
Acidification of
body fluids
body fluids
pH below 6.8 (coma)
pH below 6.2 (death)
pH below 6.2 (death)
What modern people, who are often
exposed to an environment that easily acidifies the body, need.
exposed to an environment that easily acidifies the body, need.
Alkaline foods or alkaline water
that can neutralize the body.
that can neutralize the body.
02
The size of water molecule clusters is small
Due to its small size (cluster), it is rapidly absorbed,
and it is the smallest water on Earth (50.4Hz).
and it is the smallest water on Earth (50.4Hz).
Tap water, well water,
purified water from water purifiers
purified water from water purifiers
100~150Hz
Human cells
60Hz
Hydrogen water
50.4Hz
03
Rich in minerals
Alkaline ionized water is rich in minerals.
Minerals are referred to as inorganic substances, minerals,
and electrolytes, and they are all biological components necessary
for our body to exist in balance, except for organic substances.
The minerals in alkaline ionized water are essential minerals in the water
that are ionized by electric energy, making them highly absorbable.
Minerals are referred to as inorganic substances, minerals,
and electrolytes, and they are all biological components necessary
for our body to exist in balance, except for organic substances.
The minerals in alkaline ionized water are essential minerals in the water
that are ionized by electric energy, making them highly absorbable.
04
Oxidation-Reduction Potential (ORP)
The size (cluster) of the water is small,
so it is absorbed quickly and is the smallest water (50.4Hz) on Earth.
so it is absorbed quickly and is the smallest water (50.4Hz) on Earth.

Tap water
Combining with oxygen, losing electrons,
corroding, decaying, becoming tired, aging.
corroding, decaying, becoming tired, aging.
Hydrogen water
Combining with hydrogen
Losing oxygen, Gaining electrons,
Not rusting, Fresh, Healthy, and Rejuvenating
Losing oxygen, Gaining electrons,
Not rusting, Fresh, Healthy, and Rejuvenating
Why alkaline
ionized water?
ionized water?
Ioncares started with a commitment to providing
not just clean water, but also living and healthy water.
not just clean water, but also living and healthy water.
01
Contains Alkaline
Minerals
Minerals
The essential minerals for our health
such as potassium, magnesium, calcium, sodium, etc. are ionized
and released into alkaline water, while negative ions such as
phosphorus, sulfur, chlorine, etc. are released into acidic water.
such as potassium, magnesium, calcium, sodium, etc. are ionized
and released into alkaline water, while negative ions such as
phosphorus, sulfur, chlorine, etc. are released into acidic water.


02
Ionized Water
Ordinary water is composed of 10 to 15 water molecules,
while alkaline ionized water undergoes an electrolysis process
that reduces particle size. This makes it easier for the body to absorb,
provides a smoother mouthfeel, and gives a refreshing sensation.
while alkaline ionized water undergoes an electrolysis process
that reduces particle size. This makes it easier for the body to absorb,
provides a smoother mouthfeel, and gives a refreshing sensation.
03
Ionized Alkaline
Water
Water
The human body is composed of a slightly alkaline pH
of around 7.5, and alkaline water is the water closest to the human body,
so it can help maintain the body's slightly alkaline condition.
of around 7.5, and alkaline water is the water closest to the human body,
so it can help maintain the body's slightly alkaline condition.




